Complications relating to the strategic assembly of N-heterocyclic scaffolds, and the significance of this molecular framework to the development of therapeutics, diagnostics, and materials is difficult to overstate. Likewise, the stereoselective construction of quaternary stereogenic carbons remains a long-standing challenge in synthetic chemistry. By addressing these obstacles directly, the primary objective of our research group is to broadly impact organic chemistry, materials science, and medicinal chemistry through the development of new synthetic strategies in target-directed synthesis that exploit underutilized functionality while leveraging the biphilic behavior of carbene-like intermediates to concurrently construct key C–C and C–N bonds. The main components of our program include:
- Thedevelopment of transition metal-catalyzed multicomponent couplings focused on [m+1]- and [m+3-cycloadditions for the convergent assembly of highly substituted carbon centers as a lynchpin to access translationally relevant alkaloid scaffolds.
- The validation of biological targets with the potential to treat congenital and neurodegenerative cognitive disorders through the intervention of designed CNS-active small molecules.
- Synthetic modifications of the squaraine core in the construction of designed sensing agents, and their use as a template in the development of new chiral ligands for asymmetric catalysis.
- The design of thermoresponsive ionic liquids in liquid-liquid separations to reduce the energy consumption of industrial processes (e.g., desalinations, carbon capture, battery recycling, etc.) while broadly impacting separation science.
The primary driving force behind each initiative is the need for new methods and retrosynthetic strategies to rapidly construct architecturally diverse, complex molecular architectures that are otherwise laborious or difficult to achieve using conventional approaches. The long-term goals of our group are to use these new chemical constructs in the development of improved chemotherapeutics and the design of new materials that improve the energy efficiency of industrial separations. To achieve these objectives we have established a series of interdisciplinary collaborations wherein internationally recognized expertise is leveraged to address global challenges related to climate change and public health. We are constantly on the lookout for motivated and creative postdocs, graduate, and undergraduate students excited to study synthetic organic chemistry, and make an interdisciplinary impact on the nexus between chemistry, biology, and engineering.
Exploiting Ambiphilic Reactivity in Quaternary Carbon Construction and Heterocycle Diversification.
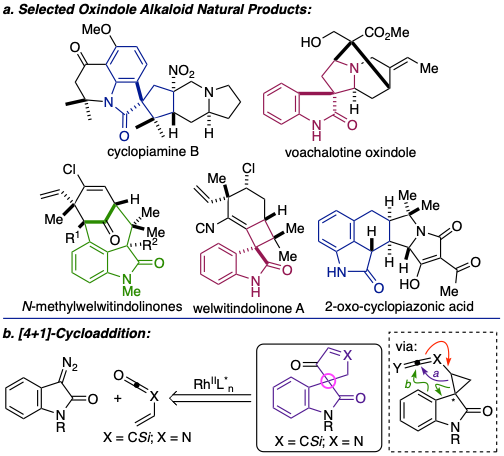
The convergent assembly of oxindole and indole frameworks in which the selective formation at C3 serves as a structural lynchpin has evolved into a major area of study in my program. Inspired by the architectural complexity and broad spectrum therapeutic potential of oxindole alkaloids, exemplified by cyclopiamine B, the welwitindolinonesand cyclopiazonic acids, we have developed complementary transition metal-catalyzed (4+1)- and (4+3)-cycloadditions employing synthetically versatile, biphilic C1 and C3 synthons to address hurdles commonly encountered in complex molecule construction. For example, our (4+1)-cycloaddition leverages the bifurcation of an intermediate spirocyclopropyl oxindole through astereocontrolled ring expansion employing diazooxindole 1 and vinyl ketene derived from cyclobutenone 2.37-39 This general design strategy resulted in the enantioselective assembly of spiro-oxindole 3.In a related study, we advanced our work on redox-driven, phosphorus-mediated C–C formations by exploiting the biphilicity of oxyphosphonium enolates to assemble 2,3-disubstituted dihydro-benzofurans and dihydroindoles through a (4+1)-cycloaddition with salicylaldehyde or 2-amino benzylchloride derivatives. This design enabled construction of oxazolones employing 1,2-dicarbonyls as biphilic C1 synthons with N-acyl isocyanates.40 In addition to the development these new methods and their application in the total synthesis of biologically relevant targets, we are interested in interrogating their mechanisms to inform the design of new catalysts and stereoselective protocols, conducted in collaboration with our colleague who specializes in molecular dynamics simulatios, Professor. Olaf Wiest (Chemistry and Biochemistry), employing variable temperature 1H/13C/31P NMR, mass spectrometry, UV-Vis spectroscopy, and other spectroscopic techniques.49
Our synthetic work toward the indole and oxindole alkaloids42 stems from our interest in the dual-specificity tyrosine-phosphorylation-regulated kinase 1A (DYRK1A), an essential kinase in cognitive development and retention, as a potential therapeutic target. The over-expression of DYRK1A has been tied to congenital and neurodegenerative cognitive disorders, which has led to a number of recent studies over the past decade toward the design of DYRK1A inhibitors. While our long-term goal is to identify a viable chemotherapeutic to treat cognitive phenotypes, our immediate primary objective is to gain a fundamental cellular understanding of the disparate outcomes associated with in vitro and in vivo DYRK1A regulation and phenotypic responses to the amelioration of DYRK1A activity. Favorable PK/PD properties and BBB permeability of many alkaloids led us to investigate the indole and oxindole scaffolds as inhibitors of DYRK1A.52 In collaboration with Professor Jeremiah Zartman (Chemical and Biomolecular Engineering), we have identified and validated in vitro viable N-heterocyclic scaffolds as potent inhibitors of DYRK1A.52
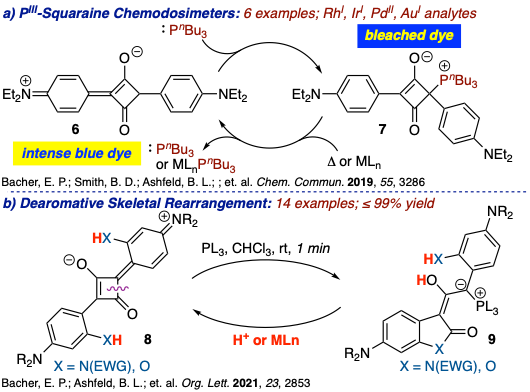
- Nucleophile-Squaraine Adducts as Chemodosimeters and Synthetic Reagents.
While squaraine dyes are best known for their chromophoric properties and use in non-invasive imaging of biological processes, nonlinear optics, photovoltaics, and ion sensing, the structural core represents a fascinating bonding motif of relatively unexplored synthetic potential. We began this area of research by exploiting the highly electrophilic cyclobutenone core of squaraine dyes, long considered an impediment to their implementation as biological sensing agents, to construct zwitterionic adducts derived from the addition of phosphines to squaraine dyes as a positive response chemodosimeter for transition metals.41 In collaboration with Professor Bradley D. Smith (Chemistry and Biochemistry), the phosphine adduct,bearing a “kite quadrilateral” core, proved exceptionally stable, and led to degrees of dye regeneration upon exposure to transition metal complexes that correlated to the electronics and coordination sphere of the metal. Extending these findings to ortho-substituted dianiline squaraine dyes resulted in a dearomative skeletal rearrangement of the squaraine core to yield oxindoles or benzofuranones.48 These ylides underwent complete retroversion to the parent dye upon exposure to a transition metal. We seek to exploit these findings in the development of chiral 1,3-squaramides derived from α-amino acids as ligands in asymmetric catalysis.
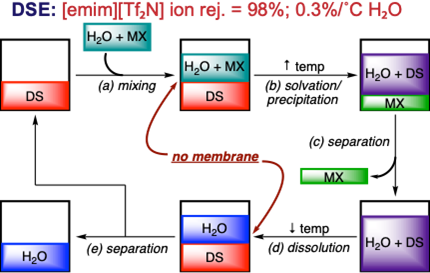
- Ionic Liquids in Energy-related Applications.
The success of worldwide efforts to minimize global energy consumption is intimately tied to the development of energy efficient separations. For over a century, the prevailing paradigm for cooling systems has relied on vapor compression using intensive electrical energy or vapor absorption into a secondary fluid, pressurized and then separated. Thermodynamics permits an alternative wherein mild sensible energy input is sufficient to drive liquid-liquid separation (LLS), an alternative to conventional heat exchange. This project has focused on the development of task-specific ionic liquids (TSILs) as thermoresponsive fluids for LLS in the development of energy efficient processes. In collaboration with, we have developed a series of ILs exhibiting lower critical solution temperature (LCST) behavior, the conversion of a homogenous solution to a biphasic mixture as temperature increases. Although the underlying chemical physics of LCST behavior is poorly understood, through a long-standing collaboration with Professor Saeed Moghaddam (University of Florida, Department of Aerospace and Mechanical Engineering) we have identified architectural parameters to predict LCST separation and examined the molecular behavior at phase transition using a combination of variable temperature MS and NMR.50 Notably, we discovered a series of LCST ILs which undergo phase separation than many of the previously reported LCST ILs (<45 ˚C). Additionally, in a collaborative effort with Professors Tengfei Luo (Department of Aerospace and Mechanical Engineering) and Edward J. Maginn (Department of Chemical and Biomolecular Engineering), we identified ILs exhibiting directional solvation that outperform existing solvents by providing a 10x increase in fresh water yield from brine with a 98% ion rejection rate in a directional solvent extraction desalination process.46 This study was recently disclosed in a recent article in Nat. Commun. that was subsequently highlighted on the ND Research homepage, which was the most accessed research article for the month of February 2021.